
【引用本文】周宗科, 黄泽宇, 杨惠林, 等. 中国骨科手术加速康复围手术期氨甲环酸与抗凝血药应用的专家共识. 中华骨与关节外科杂志, 2019, 12(2): 81-88.
中国康复技术转化及发展促进会
中国研究型医院学会关节外科学专业委员会
中国医疗保健国际交流促进会关节疾病防治分会
国家卫生计生委公益性科研专项《关节置换术安全性与效果评价》项目组
周宗科1 黄泽宇1△ 杨惠林2△ 翁习生3△ 李庭4△ 王光林1△ 张志强5△ 刘涛6△ 陈允震7* 沈慧勇8* 吴新宝4* 孙天胜9* 唐佩福10* 邱贵兴3* 裴福兴1*
(1.四川大学华西医院骨科,成都610041;2.苏州大学附属第一医院骨科,江苏苏州215006;3.中国医学科学院北京协和医学院北京协和医院骨科,北京100730;4.北京积水潭医院骨科,北京100035;5.山西医科大学第二医院骨科,太原030001;6.山东大学齐鲁医院青岛分院骨科,青岛266000;7.山东大学齐鲁医院骨科,济南250012;8.中山大学附属第八医院骨科,广州510120;9.解放军总医院第七医学中心骨科,北京100700;10.中国人民解放军总医院骨科,北京100853)
△共同第一作者
**通信作者:
陈允震,E-mail:qilucyz@yeah.net;
沈慧勇,E-mail:shenhuy@mail.sysu.edu.cn;
吴新宝,E-mail:wuxinbao_jst@126.com;
孙天胜,E-mail:suntiansheng-@163.com;
吴新宝,E-mail:wuxinbao@jst-hosp.com.cn;
余斌,E-mail:yubinol@163.com;
裴福兴,E-mail:peifuxing@vip.163.com
摘要
骨科手术出血量大,异体输血率高,极大增加了患者围手术期并发症和经济负担。氨甲环酸(TXA)是一种抗纤溶药物,大量研究均已证实其能有效减少骨科手术围手术期的失血量、降低输血率和并发症发生率。同时骨科大手术患者是静脉血栓栓塞症(VTE)的高危人群,应用抗凝血药物能有效降低VTE的发生率。如何在骨科手术患者围手术期更好地应用抗纤溶药与抗凝血药,达到既可减少患者出血量、降低输血率,同时减少患者发生VTE的风险达到止血与抗凝的平衡,保障医疗安全。通过查阅文献,中国研究型医院学会关节外科学专业委员会和中国康复技术转化及发展促进会组织专家遵循循证医学原则,经过反复讨论和通讯修改,对骨科手术加速康复围手术期抗纤溶药与抗凝血药应用方案达成共识,供广大骨科医师在临床工作中参照应用。
【关键词】骨科手术;氨甲环酸;抗凝血药;加速康复
Orthopedic surgeries are associated with a large amount of blood loss and high rate of allogeneic blood transfusion, which greatly increase the perioperative complications and economic burden of patients. Tranexamic acid (TXA) is an antifibrinolytic drug. A large number of studies have confirmed that TXA can effectively reduce the perioperative blood loss, transfusion rate and complications after orthopedic surgery. Meanwhile, patients undergoing major orthopedic surgery are at high risk of venous thrombus embolism (VTE). The use of anticoagulant drugs can effectively reduce the incidence of VTE. The ideal goal of using both TXA and anticoagulants in patients undergoing orthopedic surgery during the perioperative period is to reduce the amount of bleeding, transfusion rate and the risk of VTE in patients in order to achieve hemostasis and anticoagulation balance for medical safety. By reviewing the literatures, the Joint Surgery Specialized Committee of Chinese Research Hospital Association and the China Association of Rehabilitation Technology Transformation and Promotion organized experts to make the guideline following the principles of evidence-based medicine. After repeated discussions and communications as well as revisions, we reached the consensus on the perioperative using program of TXA and anticoagulants in patients undergoing orthopedic surgery so as to guide the daily clinical practice for a wide range of orthopedic surgeons.
【Key words】Orthopedic Surgery; Tranexamic Acid; Anticoagulant; Enhanced Recovery After Surgery
骨科手术出血量大,异体输血率高。围手术期失血量平均可达到1000~2000 ml[1],术后异体输血率达20%~80%[2],大量失血增加了患者围手术期并发症和经济负担[3]。骨科手术围手术期失血除手术切口直接出血外,由手术创伤引起的纤溶反应增强所致的失血约占总失血量的60%[4]。此外,部分骨科手术需应用止血带,由止血带引起的组织缺血再灌注损伤可进一步增强纤溶反应[5],增加出血量。
氨甲环酸(tranexamic acid, TXA)是一种抗纤溶药物,其与纤溶酶原的赖氨酸结合位点具有高亲和性,可封闭纤维蛋白结合的能力,导致纤溶活性降低,从而发挥止血作用。目前,大量研究均已证实氨甲环酸可有效减少骨科手术围手术期的失血量并降低输血率,且不增加术后静脉血栓栓塞症(venous thrombus embolism, VTE) 的发生风险[5]。
骨科大手术患者是VTE的高危人群,应用抗凝血药可有效降低VTE的发生率。在骨科手术患者围手术期更好地平衡抗纤溶药与抗凝血药的应用,既可减少患者的出血量、降低输血率,又不增加患者发生VTE的风险,保障医疗安全。国家卫生计生委公益性行业科研专项《关节置换术安全性与效果性评价》项目组(201302007)、中国研究型医院学会关节外科学专业委员会、中国康复技术转化及发展促进会和中国医疗保健国际交流促进会邀请国内专家,复习国内外52篇meta分析和400余篇论著,结合项目组26家大型医院数据库和50家推广医院数据库共2万余例病例中1万余例氨甲环酸应用经验以及全国多次氨甲环酸临床应用区域会议征求意见结果,遵循循证医学原则,对骨科手术围手术期氨甲环酸与抗凝血药应用达成共识,供广大骨科医师在临床工作中参照应用。但在应用氨甲环酸前应结合患者的全身情况,遇有不良反应及时处理。
由于骨科亚专业多,手术种类多,不同手术具有不同的特点,本共识将从关节外科、脊柱外科及创伤骨科三个方面来阐述,其他亚专业或不同手术可参照应用。
1 关节外科围手术期的氨甲环酸应用
1.1.1 静脉应用:15篇meta分析[6-8]及20篇前瞻性随机对照研究[9-11]报道,氨甲环酸给药方式主要为单次静脉滴注或多次静脉滴注,单次应用为术前10~50 mg/kg或1~3 g静脉滴注完毕;多次应用首次同单次应用,术后每间隔3~8 h给药2~4次(每次10 mg/kg或1 g)。
【推荐】①单次给药法:髋关节置换术切开皮肤前5~10 min,氨甲环酸10~50 mg/kg或1~3 g静脉滴注完毕;②多次给药法:首次给药同单次给药法,术后24 h内每间隔3~6 h给药1次(每次10 mg/kg或1 g)。
1.1.2 局部应用:研究表明,氨甲环酸局部应用能够提高局部药物浓度,减少全身吸收[12]。2篇meta分析[13,14]及8篇前瞻性随机对照研究[15-17]报道,氨甲环酸2~3 g局部应用均可以减少出血、降低输血率。目前,多数文献认为氨甲环酸较大剂量2~3 g局部应用与静脉效果相当,两者在相关并发症发生率方面无明显统计学差异。此外,目前针对氨甲环酸局部应用术后是否应该放置引流管及放置引流管是否该夹闭尚无定论,相关文献报道术后引流管夹闭时间为30 min~2 h。因此关于氨甲环酸局部应用的具体方法仍有待研究。
【推荐】髋关节置换术中氨甲环酸1~3 g 局部应用。
1.1.3 静脉和局部联合应用:研究报道,氨甲环酸在髋关节置换术围手术期静脉滴注联合局部应用相比单纯静脉滴注或局部应用能更有效减少出血、降低输血率[18,19]。具体方法为在单纯静脉应用的基础上,同时关闭切口前氨甲环酸以总量1~2 g局部应用。
【推荐】静脉方法同单纯静脉应用,联合关闭切口前氨甲环酸1~2 g局部应用。
1.2.1 静脉应用:20篇meta分析[20-31]及24篇前瞻随机对照研究报道[1,5,32-35],氨甲环酸给药方式主要为单次静脉滴注或多次静脉滴注,单次应用为术前(不应用止血带者)或松止血带前5~10 min,20~60 mg/kg或1~5 g静脉滴注完毕;多次应用首次同单次应用,术后每间隔3~4 h给药4~6次(每次10 mg/kg或1 g)。同时有2篇前瞻性随机对照研究[1,10]表明,在多次静脉应用氨甲环酸的条件下,应用止血带反而会增加围手术期的隐性失血。
【推荐】①单次给药法:膝关节置换术切开皮肤前(不应用止血带者)或松止血带前5~10 min,氨甲环酸20~60 mg/kg或1~5 g静脉滴注完毕;②多次给药法:首次给药同单次给药法,术后24 h内每间隔3~4 h给药1次(每次10 mg/kg或1 g),同时在多次给药的情况下推荐不应用止血带。
1.2.2 局部应用:6篇meta分析[7,36-38]及18篇前瞻性随机对照研究[33,39,40]报道,氨甲环酸局部应用的最低有效剂量为2 g、最低有效浓度为20 mg/ml,氨甲环酸大剂量3~5 g或高浓度>20 mg/ml局部应用能有效减少膝关节置换术围手术期出血、降低输血率。局部应用方法为关闭切口前关节腔灌注,或关闭切口后通过引流管逆行注入,或通过注射器关节腔内注射。报道中术后引流管夹闭时间为30 min~2 h,仍尚存争议,有待进一步研究。
【推荐】膝关节置换术关闭切口前后氨甲环酸≥2 g或浓度≥20 mg/ml局部应用,由于膝关节腔内容量相对较小,优先推荐应用10%氨甲环酸。
1.2.3 静脉和局部联合应用:5篇meta分析[41-45]及7篇前瞻性随机对照研究[1,39,40,46]显示,静脉滴注联合局部应用效果高于单纯静脉滴注或单纯局部用药。具体方法为在单纯静脉应用的基础上,同时关闭切口前氨甲环酸1~2 g局部应用。
【推荐】膝关节置换术切开皮肤前(不应用止血带者)或松止血带前5~10 min,氨甲环酸20~60 mg/kg或1~5 g静脉滴注完毕,术后24 h内每间隔3~4 h给药1次(每次10 mg/kg或1 g),联合关闭切口前氨甲环酸1~2 g局部应用。
根据Caprini血栓风险因素评估表[47]及《中国骨科大手术静脉血栓栓塞症预防指南》[48],接受髋、膝关节置换术患者均为深静脉血栓形成(deep vein thrombosis, DVT)的高风险患者,术后需常规使用抗凝血药物。髋、膝关节置换术围手术期应用抗纤溶药氨甲环酸后序贯应用抗凝血药,既能减少出血,又不增加VTE发生风险[3,8,49]。氨甲环酸的止血效果与其应用剂量和应用次数有关,但随着剂量或次数的增加,VTE的发生风险也可能增大[11]。理论上认为,抗凝血药物在术后应用越早、持续时间越长,患者发生VTE的风险越小,但发生出血的风险增大。为了达到抗纤溶药和抗凝血药的平衡,大部分应用氨甲环酸的患者术后6~8 h内伤口出血趋于停止,应开始应用抗凝血药物,有引流管者观察引流管无明显出血或引流管血清已分离则表明伤口出血趋于停止,之后开始应用抗凝血药;若个别患者术后仍有明显出血,可延后应用抗凝血药[1,10,11,19,32,50]。
髋、膝关节置换术后抗凝血药预防持续时间可根据患者的风险程度和下床时间及肢体功能而定,一般预防时间最短为10 d,术后VTE风险高的患者可延长至11~35 d。在应用时应注意抗凝血药的有效性和安全性,当患者出现凝血功能异常或出血事件时,应综合评价出血与血栓的风险,及时调整药物剂量或停用。
华西医院骨科关节外科自2012年开始进行围手术期氨甲环酸和抗凝血药应用方案的系列研究[3,49,51-53],目前已积累超过1万例的应用经验。围手术期应用氨甲环酸3~6次,术后6~8 h开始抗凝,基本可以达到止血与抗凝的平衡。髋关节置换术围手术期输血率从2012 年以前的7.98% 降至目前的0.98%[3,49,53],膝关节置换术围手术期输血率从2012年以前的7.32%降至目前的0.87%[3,49,53],术后DVT的发生率维持在0.09%,无肺栓塞发生[3,49,53]。
【推荐】髋、膝关节置换术加速康复围手术期应用氨甲环酸后抗凝血药推荐:①术后6~8 h或出血停止者开始应用抗凝血药;②术后8 h仍有出血倾向者,抗凝血药可延迟到术后12 h;③个别患者术后12 h仍有出血者,抗凝血药可延迟到术后24 h;④一般抗凝血药物应用0~14 d,个别患者术后VTE风险仍高可延长至15~35 d。
2 脊柱外科围手术期的氨甲环酸应用
2 篇meta 分析[54,55] 及10 篇前瞻性随机对照研究[56-59]报道,氨甲环酸给药方式主要为单次静脉滴注或多次静脉滴注,单次应用为切皮前15 min给予15~30 mg/kg或1~2 g。多次应用首次同单次应用,但后续用药方案又分为术中维持和术后按不同时间节点给药两种。术中维持方案多为1~20 mg/(kg·h)。术后按不同时间节点给药方案多为术后每间隔3~8 h给药2~3次(每次15 mg/kg或1~2 g)。
【推荐】①单次给药法:脊柱手术切开皮肤前15 min,氨甲环酸15~30 mg/kg 或1~2 g静脉滴注完毕;②持续给药维持法:首次给药同单次给药法,术中给予1~20 mg/kg·h维持;③多次间隔给药法:首次给药同单次给药法,术后每间隔3~8 h给药2~3次(每次15 mg/kg或1~2 g)。
目前针对脊柱手术中单纯局部应用氨甲环酸的文献较少[60,61]。局部应用方法为关闭切口前术区氨甲环酸浸泡,应用剂量以1 g最为常见,浸泡时间为5 min。脊柱手术应用氨甲环酸可有效降低失血量及输血率。
【推荐】脊柱手术关闭切口前术区氨甲环酸浸泡,应用剂量1 g,浸泡时间为5 min。
目前针对脊柱外科围手术期静脉和局部氨甲环酸联合应用的文献非常有限,仅一篇前瞻性随机对照研究采用氨甲环酸15 mg/kg静脉滴注联合氨甲环酸1 g局部应用,研究表明氨甲环酸静脉和局部联合应用可有效减少术中出血及术后引流量,并有效缩短患者的住院时间[59]。
【推荐】切开皮肤前15 min氨甲环酸15 mg/kg静脉滴注,联合关闭切口前给予氨甲环酸1 g局部浸泡5 min。
根据上述文献报道,在合理使用抗凝血药物的前提下,无论是静脉还是局部应用氨甲环酸并不会增加患者围手术期发生DVT的风险。因此,术前需先对患者进行血栓危险因素评估,评估方法包括Caprini 评分[47]、Autar 评分[62]、Davison 评分[63]或Padua评分[64]。Caprini静脉血栓栓塞症风险因素评估表将VTE危险因素评分分为1、2、3、5分项,包括年龄、性别、既往史、并存疾病、手术类型及手术时间等45项,每分项评分可累加。根据评分情况分为低危(0~1分)、中危(2分)、高危(3~4分)及极高危(≥5分)4个等级(表1)。Autar深静脉血栓形成风险评估表共纳入年龄、体质指数(body mass index,BMI)、活动、特殊风险、创伤风险、高危疾病和外科手术7个大项,总分为28分,根据评分情况分为低危(≤10分)、中危(11~14分)及高危(≥15分)3个等级(表2)。Caprini评分≥2分或Autar≥11分者需在基本预防及物理预防的基础上应用抗凝血药,应用时间需从术后12 h开始,存在出血风险的患者可以延迟至术后24 h,一般预防时间为10~14 d,个别VTE风险仍高者可延长至35 d。

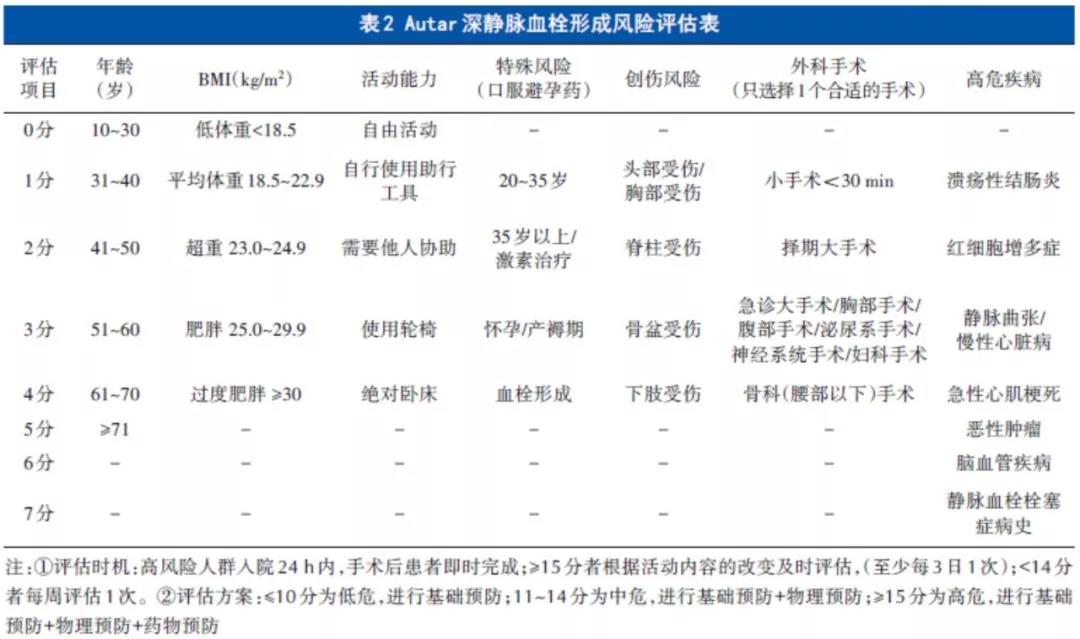
【推荐】脊柱外科加速康复围手术期应用氨甲环酸后抗凝血药推荐:①高危或以上:即Caprini评分≥3分或Autar评分≥15分的患者,术后12 h出血停止者开始应用抗凝血药,一般抗凝血药应用10~14 d,术后VTE 风险仍高的患者可延长至15~35 d;②中危:即Caprini评分=2分或Autar评分11~14分的患者,术后12~24 h出血停止者开始应用抗凝血药,一般抗凝血药应用10~14 d,个别患者VTE风险仍高抗凝血药物可延长至15~35 d;③低危:即Caprini 评分0~1 分或Autar评分≤10分的患者,可仅应用基本预防和物理预防措施。④患者存在高出血风险,抗凝血药可延迟至术后24 h或者不用抗凝血药物抗凝,采用基本预防和物理预防措施。
3 创伤骨科围手术期的氨甲环酸应用
7篇meta分析[65-68]及9篇前瞻性随机对照研究[69,70]报道,氨甲环酸给药方式主要为单次静脉滴注或多次静脉滴注。单次应用在手术切开皮肤前15~30 min,剂量为10~20 mg/kg或1~2 g。多次应用首次同单次应用,3 h后或关闭切口前追加1次(每次10~20 mg/kg或1~2 g)。文献表明在老年髋部骨折、股骨干骨折、跟骨骨折、骨盆骨折患者中应用上述方案,平均可减少300 ml失血量,使围手术期血红蛋白下降量控制在20 g/L以内,显著降低术后输血率。
【推荐】①单次给药法:切开皮肤前15~30 min给予氨甲环酸10~20 mg/kg或1~2 g静脉滴注;②多次给药法:首次给药同单次给药法,3 h后或关闭切口前追加1次(每次10~20 mg/kg或1~2 g)。
2篇meta分析[67,71]及4篇前瞻性随机对照研究[72-74]报道,氨甲环酸在髋部骨折、股骨骨折的局部应用方法为关闭切口前氨甲环酸2~3 g于筋膜下及肌肉内注射。氨甲环酸局部应用可有效降低约43%的股骨骨折输血率。
【推荐】闭合骨折手术关闭切口前氨甲环酸2~3 g局部应用,于骨折断端周围筋膜下及肌肉内注射。
目前针对创伤骨科围手术期氨甲环酸静脉和局部联合应用的文献较少,仅一篇前瞻性随机对照研究采用切开皮肤前10 min氨甲环酸1 g静脉滴注,联合关闭切口前氨甲环酸3 g筋膜下及肌肉内注射,结果显示联合应用可以显著降低股骨骨折围手术期的出血量及输血率[75]。
【推荐】切开皮肤前10 min氨甲环酸1 g静脉滴注,联合关闭切口前氨甲环酸3 g筋膜下及肌肉内注射。
创伤骨科围手术期应用抗纤溶药氨甲环酸后序贯应用抗凝血药,既能减少出血,又不增加VTE发生风险。随着氨甲环酸应用次数及剂量的增加,VTE的发生风险也会增加。文献报道应用氨甲环酸同时应采用常规抗凝血药预防VTE,研究结果表明应用氨甲环酸同时联合抗凝血药并不增加创伤骨科患者围手术期VTE 的风险。术前可采用Caprini 评分[47]或Autar 评分[62]评价患者围手术期DVT的风险,对于Caprini评分≥3分或Autar评分≥14分的患者可于术后6~8 h应用抗凝血药物,其余患者应参照《中国骨科创伤患者围手术期静脉血栓栓塞症预防的专家共识》于术后12 h常规应用抗凝血药物[76],推荐预防时间为10~35 d。
【推荐】创伤骨科加速康复围手术期应用氨甲环酸后抗凝血药推荐:①高危或以上:即Caprini评分≥3分或Autar评分≥15分的患者,术后8 h出血停止者开始应用抗凝血药,一般抗凝血药应用10~14 d,术后VTE风险仍高的患者抗凝血药物可延长至15~35 d;②中危:即Caprini评分=2分或Autar评分11~14分的患者,术后12 h出血停止者开始应用抗凝血药,一般抗凝血药应用10~14 d,个别患者术后VTE风险仍高抗凝血药物可延长至15~35 d;③低危:即Caprini评分0~1分或Autar评分≤10分的患者,可仅应用一般预防和物理预防措施。
4 纤溶指标的检测与评价
谢锦伟等[51,53]研究报道关节置换术后患者体内纤溶指标逐渐升高,术后6 h达到高峰,持续24 h后逐渐下降。纤溶是隐性失血的主要原因,术后抗纤溶药的应用能适度减缓或适度抑制纤溶,从而达到减少失血的目的[49]。通常情况下术后24 h氨甲环酸多次静脉应用适度抑制纤溶是安全的,需要氨甲环酸大量静脉应用时可检测纤溶指标如D-二聚体和纤维蛋白(原)降解产物,并注意抗凝药物的应用,达到止血与抗凝的平衡,既能减少失血又不增加血栓的风险。
附:《中国骨科手术加速康复围手术期氨甲环酸与抗凝血药应用的专家共识》专家委员会成员(按姓氏笔划排序)
王光林 王俊文 王爱国 方 跃
尹宗生 吕 刚 朱庆生 刘 涛
刘 浩 许伟华 许 杰 许建中
孙天胜 严世贵 李 庭 李 锋
杨惠林 吴新宝 邱贵兴 余 斌
沈建雄 沈惠林 张志强 张怡元
张晓岗 张 峰 陈允震 陈 仲
尚希福 岳 辰 金群华 周宗科
郑 稼 赵光辉 胡旭栋 胡懿郃
夏亚一 钱齐荣 翁习生 唐佩福
黄 伟 黄泽宇 黄 强 盛加根
康鹏德 屠重棋 彭 昊 谢锦伟
裴福兴 潘志军
参考文献
[1] Huang Z, Xie X, Li L, et al. Intravenous and topical tranexamic acid alone are superior to tourniquet use for primary total knee arthroplasty: a prospective, randomized controlled trial. J Bone Joint Surg Am, 2017, 99(24): 2053-2061.
[2] Chen AF, Klatt BA, Yazer MH, et al. Blood utilization after primary total joint arthroplasty in a large hospital network. HSS J, 2013, 9(2): 123-128.
[3] Huang Z, Huang C, et al. Analysis of a large data set to identify predictors of blood transfusion in primary total hip and knee arthroplasty. Transfusion, 2018, 58(8): 1855-1862.
[4] Liu X, Zhang X, Chen Y, et al. Hidden blood loss after total hip arthroplasty. J Arthroplasty, 2011, 26(7): 1100-1105.
[5] Engel JM, Hohaus T, Ruwoldt R, et al. Regional hemostatic status and blood requirements after total knee arthroplasty with and without tranexamic acid or aprotinin. Anesth Analg, 2001, 92(3): 775-780.
[6] Moskal JT, Capps SG. Meta-analysis of intravenous tranexamic acid in primary total hip arthroplasty. Orthopedics, 2016, 39(5): e883-892.
[7] Chen Y, Chen Z, Cui S, et al. Topical versus systemic tranexamic acid after total knee and hip arthroplasty: A meta-analysis of randomized controlled trials. Medicine (Baltimore), 2016, 95(41): e4656.
[8] Xie J, Hu Q, Huang Q, et al. Comparison of intravenous versus topical tranexamic acid in primary total hip and knee arthroplasty: an updated meta-analysis. Thromb Res, 2017, 153: 28-36.
[9] Clave A, Fazilleau F, Dumser D, et al. Efficacy of tranexamic acid on blood loss after primary cementless total hip replacement with rivaroxaban thromboprophylaxis: a casecontrol study in 70 patients. Orthop Traumatol Surg Res, 2012, 98(5): 484-490.
[10] Lei Y, Huang Q, Huang Z, et al. Multiple-dose intravenous tranexamic acid further reduces hidden blood loss after total hip arthroplasty: a randomized controlled trial. J Arthroplasty, 2018, 33(9): 2940-2945.
[11] Xie J, Hu Q, Ma J, et al. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss and the inflammatory response following enhanced-recovery primary total hip arthroplasty: a randomised clinical trial. Bone Joint J, 2017, 99-B(11): 1442-1449.
[12] Imai N, Dohmae Y, Suda K, et al. Tranexamic acid for reduction of blood loss during total hip arthroplasty. J Arthroplasty, 2012, 27(10): 1838-1843.
[13] Chen S, Wu K, Kong G, et al. The efficacy of topical tranexamic acid in total hip arthroplasty: a meta-analysis. BMC Musculoskelet Disord, 2016, 17: 81.
[14] Wang C, Xu GJ, Han Z, et al. Topical application of tranexamic acid in primary total hip arthroplasty: a systemic review and meta-analysis. Int J Surg, 2015, 15: 134-139.
[15] Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty, 2013, 28(9): 1473-1476.
[16] Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty, 2014, 29(11): 2113-2116.
[17] Yue C, Kang P, Yang P, et al. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty, 2014, 29 (12): 2452-2456.
[18] Liu X, Liu J, Sun G. A comparison of combined intravenous and topical administration of tranexamic acid with intravenous tranexamic acid alone for blood loss reduction after total hip arthroplasty: A meta-analysis. Int J Surg, 2017, 41: 34-43.
[19] 岳辰, 谢锦伟, 蔡东峰, 等. 静脉联合局部应用氨甲环酸减少初次全髋关节置换术围手术期失血的有效性及安全性研究. 中华骨与关节外科杂志, 2015, 8(1): 44-48.
[20] Ho KM, Ismail H. Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care, 2003, 31(5): 529-537.
[21] Fu DJ, Chen C, Guo L, et al. Use of intravenous tranexamic acid in total knee arthroplasty: a meta-analysis of randomized controlled trials. Chin J Traumatol, 2013, 16(2): 67-76.
[22] Tan J, Chen H, Liu Q, et al. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res, 2013, 184(2): 880-887.
[23] Wu Q, Zhang HA, Liu SL, et al. Is tranexamic acid clinically effective and safe to prevent blood loss in total knee arthroplasty? A meta-analysis of 34 randomized controlled trials. Eur J Orthop Surg Traumatol, 2015, 25(3): 525-541.
[24] Wang H, Shen B, Zeng Y. Comparison of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled and prospective cohort trials. Knee, 2014, 21(6): 987-993.
[25] Chen X, Zhu X, Yang S, et al. Tranexamic Acid Treatment Decreases Hidden Blood Loss in Total Knee Arthroplasty. Am J Ther, 2016, 23(6): e1397-e1405.
[26] He P, Zhang Z, Li Y, et al. Efficacy and safety of tranexamic acid in bilateral total knee replacement: a meta-analysis and systematic review. Med Sci Monit, 2015, 21: 3634-3642.
[27] Shin YS, Yoon JR, Lee HN, et al. Intravenous versus topical tranexamic acid administration in primary total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc, 2017, 25(11): 3585-3595.
[28] Jiang X, Ma XL, Ma JX. Efficiency and safety of intravenous tranexamic acid in simultaneous bilateral total knee arthroplasty: a systematic review and meta-analysis. Orthop Surg, 2016, 8(3): 285-293.
[29] Fu Y, Shi Z, Han B, et al. Comparing efficacy and safety of 2 methods of tranexamic acid administration in reducing blood loss following total knee arthroplasty: a meta-analysis. Medicine
(Baltimore), 2016, 95(50): e5583.
[30] Wu Y, Yang T, Zeng Y, et al. Tranexamic acid reduces blood loss and transfusion requirements in primary simultaneous bilateral total knee arthroplasty: a meta-analysis of randomized controlled trials. Blood Coagul Fibrinolysis, 2017, 28(7): 501-508.
[31] Dai WL, Zhou AG, Zhang H, et al. Most effective regimen of tranexamic acid for reducing bleeding and transfusions in primary total knee arthroplasty: a meta-analysis of randomized controlled trials. J Knee Surg, 2018, 31(7): 654-663.
[32] 胡旭栋, 周宗科, 裴福兴, 等. 全膝关节置换围手术期氨甲环酸不同使用方法的有效性和安全性. 中华骨科杂志, 2014, 34(6): 599-604.
[33] Yuan X, Li B, Wang Q, et al. Comparison of 3 routes of administration of tranexamic acid on primary unilateral total knee arthroplasty: a prospective, randomized, controlled study. J Arthroplasty, 2017, 32(9): 2738-2743.
[34] Lee SY, Chong S, Balasubramanian D, et al. What is the ideal route of administration of tranexamic acid in TKA? A randomized controlled trial. Clin Orthop Relat Res, 2017, 475 (8): 1987-1996.
[35] Li ZJ, Zhao MW, Zeng L. Additional dose of intravenous tranexamic acid after primary total knee arthroplasty further reduces hidden blood loss. Chin Med J (Engl), 2018, 131(6): 638-642.
[36] Zhao-Yu C, Yan G, Wei C, et al. Reduced blood loss after intra-articular tranexamic acid injection during total knee arthroplasty: a meta-analysis of the literature. Knee Surg Sports Traumatol Arthrosc, 2014, 22(12): 3181-3190.
[37] Yue C, Pei F, Yang P, et al. Effect of topical tranexamic acid in reducing bleeding and transfusions in TKA. Orthopedics, 2015, 38(5): 315-324.
[38] Chen TP, Chen YM, Jiao JB, et al. Comparison of the effectiveness and safety of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled trials. J Orthop Surg Res, 2017, 12(1): 11.
[39] Lin SY, Chen CH, Fu YC, et al. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty, 2015, 30(5): 776-780.
[40] Song EK, Seon JK, Prakash J, et al. Combined administration of Ⅳ and topical tranexamic acid is not superior to either individually in primary navigated TKA. J Arthroplasty, 2017, 32(1): 37-42.
[41] Shang J, Wang H, Zheng B, et al. Combined intravenous and topical tranexamic acid versus intravenous use alone in primary total knee and hip arthroplasty: A meta-analysis of randomized controlled trials. IntJSurg, 2016, 36(PtA): 324-329.
[42] Zhang XQ, Ni J, Ge WH. Combined use of intravenous and topical versus intravenous tranexamic acid in primary total joint arthroplasty: a meta-analysis of randomized controlled trials. Int J Surg, 2017, 38: 15-20.
[43] Lin C, Qi Y, Jie L, et al. Is combined topical with intravenous tranexamic acid superior than topical, intravenous tranexamic acid alone and control groups for blood loss controlling after total knee arthroplasty: a meta-analysis. Medicine (Baltimore), 2016, 95(51): e5344.
[44] Mi B, Liu G, Lv H, et al. Is combined use of intravenous and intraarticular tranexamic acid superior to intravenous or intraarticular tranexamic acid alone in total knee arthroplasty? A meta-analysis of randomized controlled trials. J Orthop Surg Res, 2017, 12(1): 61.
[45] Peng Zhang MM, Jifeng Li MM, Xiao Wang MM. Combined versus single application of tranexamic acid in total knee and hip arthroplasty: A meta-analysis of randomized controlled trials. Int J Surg, 2017, 43: 171-180.
[46] Huang Z, Ma J, Shen B, et al. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty, 2014, 29(12): 2342-2346.
[47] Golemi I, Adum JPS, Tafur A, et al. Venous thromboembolism prophylaxis using the Caprini score. Dis Mon, 2019, doi: 10.1016/j.disamonth.2018.12.005. [Epub ahead of print]
[48] 中华医学会骨科学分会. 中国骨科大手术静脉血栓栓塞症预防指南. 中华骨科杂志, 2016, 36(2): 65-71.
[49] Xie J, Hu Q, Huang Z, et al. Comparison of three routes of administration of tranexamic acid in primary unilateral total knee arthroplasty: Analysis of a national database. Thromb Res, 2019, 173: 96-101.
[50] 岳辰, 康鹏德, 沈彬, 等. 氨甲环酸用于首次髋关节置换术的系统评价和Meta分析. 中国矫形外科杂志, 2013, 21(12): 1167-1172.
[51] 谢锦伟, 姚欢, 岳辰, 等. 初次髋、膝关节置换术后纤溶变化. 中国矫形外科杂志, 2016, 24(10): 931-936.
[52] Cao G, Huang Z, Huang Q, et al. Incidence and risk factors for blood transfusion in simultaneous bilateral total joint arthroplasty: a multicenter retrospective study. J Arthroplasty, 2018, 33(7): 2087-2091.
[53] 谢锦伟, 岳辰, 马俊, 等. 初次髋膝关节置换术后静脉血栓发生情况的观察比较. 中国骨伤, 2016, 29(8): 708-712.
[54] Cheriyan T, Maier SP, 2nd, Bianco K, et al. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J, 2015, 15(4): 752-761.
[55] Gong M, Liu G, Chen L, et al. The efficacy and safety of intravenous tranexamic acid in reducing surgical blood loss in posterior lumbar interbody fusion for the adult: a systematic review and a meta-analysis. World Neurosurg, 2018, doi: 10.1016/j.wneu.2018.09.115. [Epub ahead of print]
[56] Wang Q, Liu J, Fan R, et al. Tranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial. Eur Spine J, 2013, 22(9): 2035-2038.
[57] Shi H, Ou Y, Jiang D, et al. Tranexamic acid reduces perioperative blood loss of posterior lumbar surgery for stenosis or spondylolisthesis: a randomized trial. Medicine (Baltimore), 2017, 96(1): e5718.
[58] Kim KT, Kim CK, Kim YC, et al. The effectiveness of lowdose and high-dose tranexamic acid in posterior lumbar interbody fusion: a double-blinded, placebo-controlled randomized study. Eur Spine J, 2017, 26(11): 2851-2857.
[59] Ou Y, Wei J, Li R, et al. Clinical research of combined intravenous administration and topical application of tranexamic acid to a surgical wound during posterior lumbar fusion. Surg Innov, 2018, 25(2): 128-135.
[60] Xu D, Zhuang Q, Li Z, et al. A randomized controlled trial on the effects of collagen sponge and topical tranexamic acid in posterior spinal fusion surgeries. J Orthop Surg Res, 2017, 12(1): 166.
[61] Ren Z, Li S, Sheng L, et al. Topical use of tranexamic acid can effectively decrease hidden blood loss during posterior lumbar spinal fusion surgery: A retrospective study. Medicine (Baltimore), 2017, 96(42): e8233.
[62] Wallis M, Autar R. Deep vein thrombosis: clinical nursing management. Nurs Stand, 2001, 15(18): 47-54.
[63] Davison SP, Venturi ML, Attinger CE, et al. Prevention of venous thromboembolism in the plastic surgery patient. Plast Reconstr Surg, 2004, 114(3): 43E-51E.
[64] Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost, 2010, 8(11): 2450-2457.
[65] Xiao C, Zhang S, Long N, et al. Is intravenous tranexamic acid effective and safe during hip fracture surgery? An updated meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg, 2019, doi: 10.1007 / s00402-019-03118-6. [Epub ahead of print]
[66] Zhu Q, Yu C, Chen X, et al. Efficacy and safety of tranexamic acid for blood salvage in intertrochanteric fracture surgery: a meta-analysis. Clin Appl Thromb Hemost, 2018, 24(8): 1189-1198.
[67] Wang W, Yu J. Tranexamic acid reduces blood loss in intertrochanteric fractures: A meta-analysis from randomized controlled trials. Medicine (Baltimore), 2017, 96(52): e9396.
[68] Zhang P, He J, Fang Y, et al. Efficacy and safety of intravenous tranexamic acid administration in patients undergoing hip fracture surgery for hemostasis: a meta-analysis. Medicine (Baltimore), 2017, 96(21): e6940.
[69] Lei J, Zhang B, Cong Y, et al. Tranexamic acid reduces hidden blood loss in the treatment of intertrochanteric fractures with PFNA: a single-center randomized controlled trial. J Orthop Surg Res, 2017, 12(1): 124.
[70] Xie B, Tian J, Zhou DP. Administration of tranexamic acid reduces postoperative blood loss in calcaneal fractures: a randomized controlled trial. J Foot Ankle Surg, 2015, 54(6): 1106-1110.
[71] Zhang P, Bai J, He J, et al. A systematic review of tranexamic acid usage in patients undergoing femoral fracture surgery. Clin Interv Aging, 2018, 13: 1579-1591.
[72] Drakos A, Raoulis V, Karatzios K, et al. Efficacy of local administration of tranexamic acid for blood salvage in patients undergoing intertrochanteric fracture surgery. J Orthop Trauma, 2016, 30(8): 409-414.
[73] Jordan M, Aguilera X, Gonzalez JC, et al. Prevention of postoperative bleeding in hip fractures treated with prosthetic replacement: efficacy and safety of fibrin sealant and tranexamic acid. A randomised controlled clinical trial (TRANEXFER study). Arch Orthop Trauma Surg, 2018, doi: 10.1007/s00402-018-3089-4. [Epub ahead of print]
[74] Gillespie R, Shishani Y, Joseph S, et al. Neer Award 2015: A randomized, prospective evaluation on the effectiveness of tranexamic acid in reducing blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg, 2015, 24(11): 1679-1684.
[75] Watts CD, Houdek MT, Sems SA, et al. Tranexamic acid safely reduced blood loss in hemi- and total hip arthroplasty for acute femoral neck fracture: a randomized clinical trial. J Orthop Trauma, 2017, 31(7): 345-351.
[76] 中华医学会骨科学分会创伤骨科学组. 中国骨科创伤患者围手术期静脉血栓栓塞症预防的专家共识. 中华创伤骨科杂志, 2012, 14(6): 461-463.
本文来源: 中华骨与关节外科杂志













